Abstract
Introduction
Mature T cell lymphomas are aggressive, treatment resistant cancers that are associated with poor prognosis. Clinical application of immunotherapeutic approaches has been limited by a lack of target antigens that discriminate malignant from healthy T cells. Unlike B cell depletion, pan-T cell aplasia is prohibitively toxic. Previously we reported a targeting strategy based on the mutually exclusive expression of T cell receptor beta-chain constant domains 1 and 2 (TRBC1 and TRBC2). We identified an antibody with unique TRBC1 specificity and demonstrated that anti TRBC1 chimeric antigen receptor (CAR) T cells can ablate cells expressing TRBC1 TCRs while sparing those expressing TRBC2 TCRs. A phase I clinical study investigating the efficacy of our TRBC1 CAR is ongoing.
T cell malignancies are clonal, and the ratio of TRBC2 to TRBC1 expressing lymphoma cases is predicted to be approximately 2:1. To treat all cases of T cell lymphoma, a CAR that targets TRBC2 is needed. TRBC1 and 2 are highly homologous. Structural studies suggest that amino acid inversions at positions 4 and 5 of the constant beta chain provide an accessible discriminating portion between the two proteins.
Given the structural similarities between TRBC1 and TRBC2 and our characterized binder against TRBC1, we explored generating antibodies with specificity towards TRBC2 via a structure guided computational biology approach; engineering the previously identified TRBC1 antibody and reversing its specificity such that it recognised TRBC2.
Results
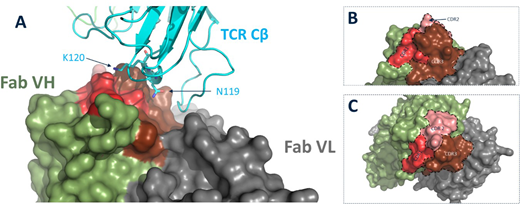
The crystal structure of the TRBC1 specific monoclonal antibody was solved in complex with a TRBC1-TCR to 2.4Å, Figure 1. Through computational biology and protein engineering we rationally designed a mutant version of TRBC1 binder that was specific for TRBC2 and had a 1000 fold decreased affinity towards TRBC1. Flow cytometry analysis of the TRBC2 specific antibody demonstrated the ability to bind to T-cells expressing TRBC2 TCRs. We further showed that the engineered antibody retained favourable biophysical characteristics with high stability (Fab Tm > 65oC) and low aggregation propensity (>99% monomer).
We used the engineered monoclonal antibody to generate a 2nd generation anti-TRBC2 CAR. We demonstrated that our anti-TRBC2 CAR showed specificity, cytokine release and cytotoxicity in 72hr co-cultures against TRBC2+ cell lines but not TRBC1+ cell lines or cell lines that did not express TCR on the surface. Anti-TRBC2 CAR T-cells also demonstrated proliferative capacity in long-term co-culture assays.
Conclusions
We have utilised structural biology and rational protein design to generate CAR-T cells capable of specifically targeting TRBC2. The combination of TRBC1 and 2 targeting CAR-T cell products with a patient stratification companion diagnostic assay offers a therapeutic strategy for the treatment of a wide range of, otherwise untreatable, T-cell lymphomas.
Figure 1. A. Structural interface between TCR Beta and Fab fragment of TRBC1 specific antibody. B and C. CDR fold of TRBC1 binder and 90o rotation.
Onuoha:Autolus Ltd: Employment, Equity Ownership, Patents & Royalties. Ferrari:Autolus Ltd: Employment, Equity Ownership. Bulek:Autolus Ltd: Employment, Equity Ownership, Patents & Royalties. Bughda:Autolus Ltd: Employment, Equity Ownership, Patents & Royalties. Manzoor:Autolus Ltd: Employment, Equity Ownership, Patents & Royalties. Srivastava:Autolus Ltd: Employment, Equity Ownership. Ma:Autolus Ltd: Employment. Karattil:Autolus Ltd: Employment, Equity Ownership, Patents & Royalties. Kinna:Autolus Ltd: Employment, Equity Ownership. Thomas:Autolus Ltd: Employment, Equity Ownership, Patents & Royalties. Cordoba:Autolus Ltd: Employment; Autolus Ltd: Patents & Royalties; Autolus Ltd: Equity Ownership. Maciocia:Autolus: Equity Ownership, Patents & Royalties: UCLB. Pule:Autolus Ltd: Employment, Equity Ownership, Other: Salary contribution paid for by Autolus, Research Funding; University College London: Patents & Royalties: Patent with rights to Royalty share through UCL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal